SACHA: a Pediatric Cancer Registry
SACHA (Secured Access to innovative medicines for CHildren, adolescents and young adults with cAncer) is an international observational registry coordinated by Gustave Roussy. It aims to secure access to innovative anticancer medicines for children, adolescents and young adults (AYA) outside of clinical trials, while collecting real-world evidence (RWE) of safety and efficacy to inform future research and regulatory decisions.
Launched in France in 2020, SACHA expanded to multiple countries from 2023 onwards thanks to the support of Fight Kids Cancer, and now brings together pediatric oncology centres committed to securing access to innovation.
Rationale & objectives of the SACHA Study
SACHA was developed to address a critical need in pediatric oncology: generating robust, real-world evidence of innovative therapies used under compassionate or off-label access.
The project has several key goals:
- Securing early access to innovative anticancer treatments outside traditional trials
- Collecting structured real-world data (RWD) on safety and potential efficacy
- Identifying early efficacy signals that can shape future clinical trials
- Supporting regulatory and ethical frameworks for compassionate use programs
- Fostering international collaboration, enabling pediatric oncology centers to share knowledge and collectively advance therapeutic innovation
Methodology of an international observational registry
SACHA is a prospective, non-interventional, international registry designed to monitor innovative anticancer therapies administered to patients aged 0–25 years outside clinical trials. Inclusion is based on a compassionate or off-label use of an innovative anticancer therapy validated by a multidisciplinary tumor board.
For each therapy interest clinical data is analyzed by by national coordinators in each participating country, while the Pharmacovigilance Team at Gustave Roussy remotely reviews all ≥2 clinical or ≥3 biological related adverse drug reactions using CTCAE version 5. For hematological events, only transfusion-requiring thrombocytopenia/anemia or grade 4 neutropenia lasting ≥7 days. Generated CIOMS forms are then transmitted to the national coordinating center, ensuring harmonized reporting and patient safety oversight.
SACHA is officially recognized as a real-world data source by the French National Authority for Health (HAS) and is strongly supported by the French National Agency for the Safety of Medicines and Health Products (ANSM). It is listed in the HMA-EMA catalogue of real-world data sources and is backed by leading organizations in pediatric oncology, including the French Society of Pediatric Oncology (SFCE) and the Innovative Therapies for Children and Adolescents with Cancer Consortium (ITCC).
SACHA eligibility criteria
SACHA Patients
- Patients are ≤ 25 years-old with a recurrent/ refractory pediatric malignancy
- Patients are not eligible in a clinical trial or with no therapeutic option available in an ongoing trial
- Patients are treated with a new anticancer medicine through either off-label or compassionate use
- SACHA treatments are recommended by a multidisciplinary tumor board.

SACHA therapies of interest
- Any innovative drug through compassionate use
- Any anticancer medicine with a first European Marketing Authorisation after 2007 used off-label
Progress to Date
Opening of the study in France
Inclusion of the first patient in Austria
Inclusion of the first patient in Spain
Publication of the landmark SACHA-France study article.
Inclusion of the first patient in the UK
Inclusion of the first patient in the Denmark
Publication of the first ancillary study linked to SACHA
Inclusion of the first patient in Italy
Launch of the SACHA International website
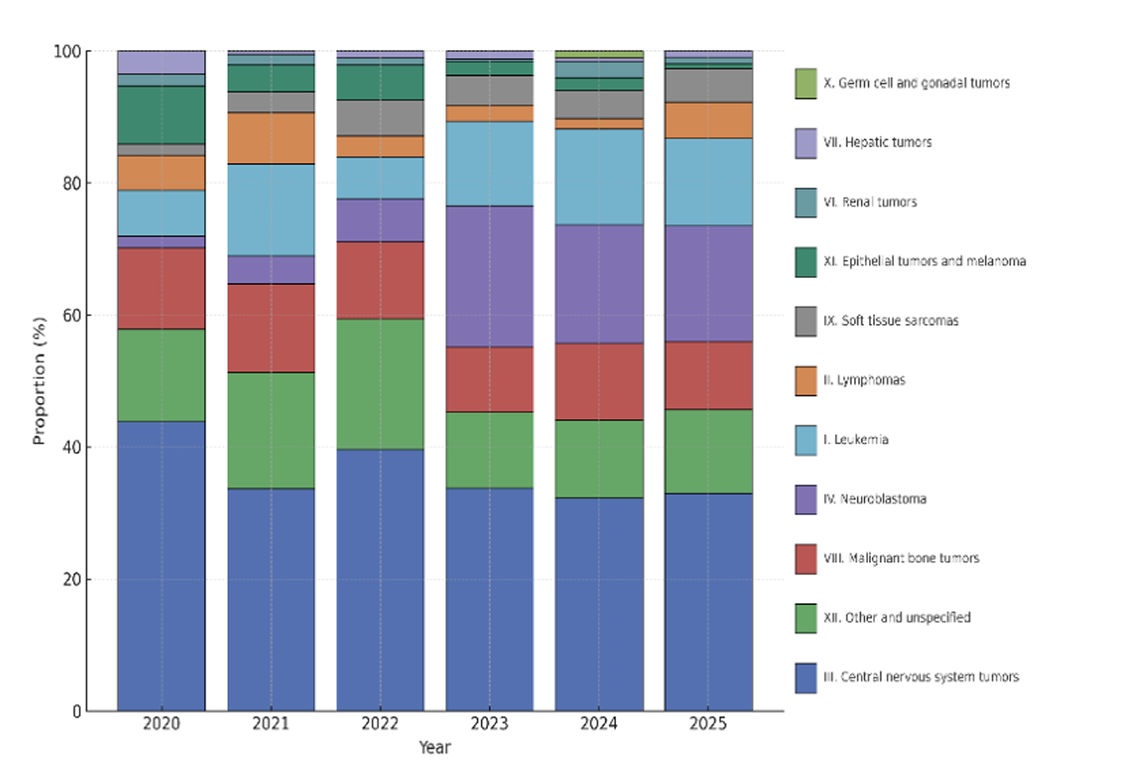
SACHA-France proof-of-concept
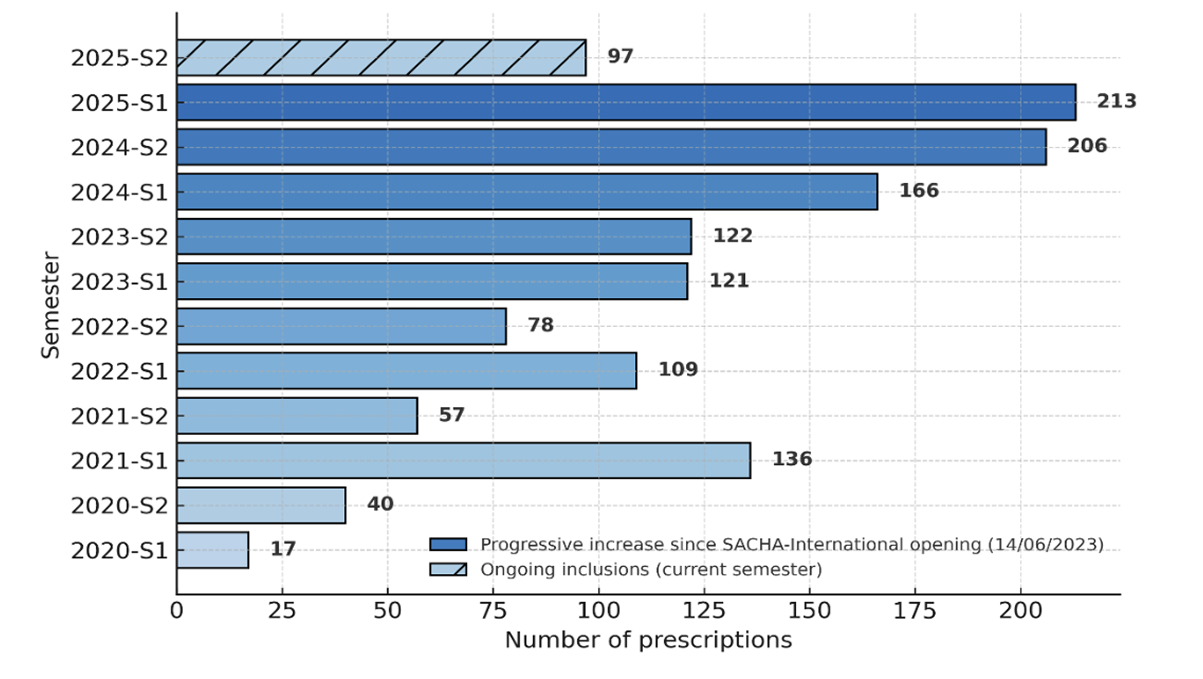
Prescriptions number per year
114 prescriptions
187 prescriptions
193 prescriptions
57 prescriptions
as of July 2025
monitored
SACHA international
Prescriptions number per semester